
Recently, the research result, “Photo-induced iron-catalyzed synthesis of amides via nitro compounds and chlorinated alkanes”, of Professor Zhang Fanglin’s group was published in Green Chemistry, a leading international journal in the field of chemistry, with Zhang Qunliang, a doctoral student of WUT, and Liu Wenxin, a postgraduate student of WUT being co-first authors, and Prof. Zhang Fanglin of WUT and Prof. Zhou Yirong from Huazhong University of Science and Technology (HUST) being corresponding authors. The research work was supported by the National Natural Science Foundation of China.
Amide compounds are common chemical backbones in the synthetic community and are widely used in natural products, medicine, biology and materials science. Meanwhile, amide compounds are also common chemical structures in catalysts and play important roles as ligands or organic small molecule catalysts in the field of catalysis. Therefore, it is of great significance to construct amides using simple and effective methods. However, most of the common amides are synthesized by carboxylic acid pre-activation or using condensation reagents, and the green synthesis of amides remains a great challenge. Zhang Fanglin 's group has made new progress in this research area.
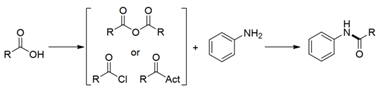
Figure 1 Classical synthesis of amides (Source: Green Chemistry)
Zhang Fanglin 's group has long been working in the field of photocatalysis and metal catalysis. For the green synthesis of N-aryl amides, a series of N-aryl amides are designed to be efficiently and rapidly synthesized under environmental-friendly and simple conditions by visible-light-induced iron-catalysis using commercially inexpensive and abundant nitroaromatics and chloroalkanes as starting materials. The method has high atom economy, good functional group tolerance, and needs no additional reducing agents and photocatalysts.
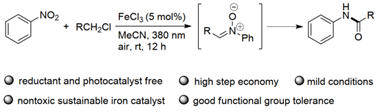
Figure 2 Photo-induced iron-catalyzed synthesis of amides via nitro compounds and chlorinated alkanes (Source: Green Chemistry)
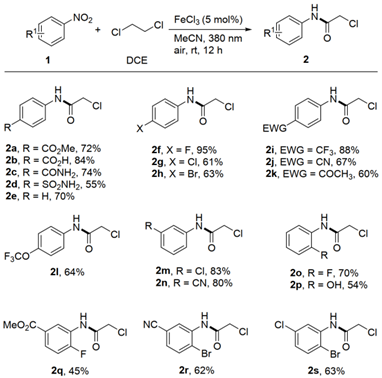
Figure 3 Preparation of chlorinated alkanes by reaction of nitroaromatics with DCE (Source: Green Chemistry)
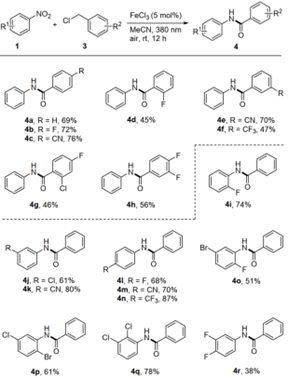
Figure 4 Preparation of aryl amides by reaction of nitroaromatics with benzyl chloride (Source: Green Chemistry)

Figure 5 Possible reaction mechanisms (Source: Green Chemistry)
Zhang Fanglin 's group realized the green synthesis of N-aryl amides with efficient and mild reaction conditions to construct a series of amide products. The key to the success of this method is the use of a photoinduced/iron-catalyzed reaction mode. The unique reaction properties of nitro compounds under photo/iron conditions were confirmed. To provide a theoretical and practical basis for the application of nitro compounds in the field of photochemistry, it also contributes to the synthesis method of amides.
Rewritten by: YuMengmei
Reviewed by: Han Tongyuan
Edited by: Li Tiantian
Source: School of Chemistry, Chemical Engineering and Life Science
|
|